HTML
-
全世界范围内,超过1/5的原油被细菌破坏或改造[1]。1969年Winter et al.[2]首次将自然条件下正构烷烃的消耗解释为原油生物降解作用后,生物降解的概念被定义并在全球广泛应用。国内外学者对生物降解作用进行了大量的研究发现,生物的降解作用是一个准阶梯式的过程,饱和烃中的类异戊二烯、姥鲛烷和植烷会在正构烷烃被消耗后发生降解,随后生物降解会破坏原油中的规则甾烷和烷基化芳香族化合物,然后依次是藿烷、芳香甾族烃类、重排甾烷、三环萜烷,且在高级阶段,似乎生成某些生物标志化合物,如25-降藿烷和断藿烷[3⁃4]。而在生物降解过程中,各生物标志化合物系列又有一定的降解顺序,比如在严重生物降解原油中,甾烷系列被降解或被蚀耗的先后顺序为:ααα20R>αββ20R>αββ20S≥ααα20S,C27>C29>C28规则甾烷>重排甾烷,C27,C28,C29甾烷>C20,C21甾烷(式中>表示优先降解);C31和C32升藿烷对生物降解的易感性比C30藿烷强,而升藿烷系列中高碳数的藿烷同系物具有优先抵御生物降解的能力,尤其是C35五升藿烷[5⁃8]。一般认为的芳烃序列为烷基苯、萘、苯并噻吩、菲、二苯并噻吩,其抗生物降解的能力随着芳环数和烷基取代基数的增加而增加[9⁃10]。
Anders et al.[11]和Callegos[12]1971年在Uinta绿河页岩中首次发现C20-C25三环萜烷以来,有关三环萜烷的研究也引起人们的关注。随后在1983年Moldowan et al.[13]通过离子跃迁分析从石油样品中识别了C19-C45三环萜烷系列的化合物,后来学者又将此系列化合物的识别扩展到C54[14]。与此同时,国内学者开始将三环萜烷化合物应用到原油的生源输入及油源对比的研究[15⁃16]。1979年,Seifert et al.[17]通过在实验室模拟自然发生的生物降解过程,证实了三环萜烷可以在大多数严重降解的情况下存活,随后不断有学者证明三环萜烷是抗生物降解能力较强的化合物之一[18⁃21],其中Lin et al.[21]在1989年发现三环萜烷可能发生蚀变的等级与重排甾烷持平,生物降解等级大于8级。
近年来,国内外学者关于生物降解作用对原油的影响以及生物降解原油中生物标志化合物的特征研究越来越多。Bost et al.[22]在喜氧细菌富集培养物实验中发现C28三环萜烷会先于C29三环萜烷降解;马遵敬[23]研究哈山地区二叠系原油时发现低碳数三环萜烷分子抗生物降解能力可能更强;程熊等[24]研究得出三环萜烷系列化合物(除C20三环萜烷以外)抗生物降解能力随碳数的增加而增强;Huang et al.[25]则得出一些严重生物降解样品中C26三环萜烷是唯一存在的三环萜烷系列化合物。然而,针对三环萜烷系列化合物内组成与原油生物降解程度之关系的文献鲜有报道。基于前人研究,笔者以准噶尔盆地西北缘生物降解的油砂为例,重点探讨了遭受强烈生物降解作用原油中三环萜烷化合物分布与组成特征,提出了一些评价原油遭受生物降解程度比值,为遭受强烈生物降解作用原油的地区油气勘探提供地球化学依据。
-
准噶尔盆地油砂主要分布在盆地边缘,沿盆地或凹陷外缘富集和展布。迄今为止,西北缘是准噶尔盆地油气最多、油气藏规模最大的地区,盆地深层至浅层分布着常规原油—稠油—油砂。自东北向西南方向延伸约200 km,包括夏子街、风城、乌尔禾、百口泉、克拉玛依、红山嘴、小拐、车排子等油田,各油田之间的物性变化较大,有正常原油,也有大量的稠油以及少量的轻质油[26⁃27]。风城油砂矿区是西北缘最大的油砂矿区,地层较为简单, 自下而上为石炭系(C)、上侏罗统齐古组(J3q)或缺失,下白垩统吐谷鲁组(K1t)、第四系(Q)四套地层。油砂主要赋存于下白垩统吐谷鲁组、上侏罗统齐古组。
准噶尔盆地属于山间型中间地块盆地,在地质历史演化过程中,它又是一个大型的复合叠加盆地。在盆地的地史演化过程中,西北缘构造活动主要表现为逆掩断裂,形成延伸250 km的推覆体构造带。推覆体构造带位于中央生油坳陷区的西北侧,与玛湖生油坳陷相邻,丰富的油源为油气藏提供了物质基础;长期的断裂活动、频繁的地层超覆、不整合为油气运移提供了良好的通道,断裂带的隐伏性,为油气聚集提供了较好的圈闭条件。因此,风城区齐古组、吐谷鲁组油藏形成于不整合面之上的超覆尖灭带属于稠油油藏。
研究表明,准噶尔盆地西北缘具有多期次成藏的特征,第一次成藏时间较早,主要是三叠纪末期的印支运动使西北缘逆掩断裂带剧烈活动,丰富的油气运移形成了二叠系—三叠系的早期油藏;第二次是侏罗纪—白垩纪时期的燕山运动,使西北缘造成齐古组的不整合,从而使印支期形成的油藏遭受破坏,沿不整合面发生第二次运移,形成齐古组油藏;燕山运动晚期活动强度减弱,西北缘在盆地中心沉降过程中,相对速度较慢,出现了吐古鲁组的超覆不整合及局部地段的微弱断裂活动,促使油气第三次运移,形成了吐古鲁组油藏和地面大面积油砂。据地化指标分析对比,不论浅层深层,稠油稀油,均具有较为一致的特性,油源来自玛湖凹陷,其推覆构造各部位油藏皆为同源原油[28]。
-
风城地区的油砂与稠油具有伴生的关系,本文主要分析了风城地区风砂51井、风砂52井、风砂53井、风砂54井、风砂55井、风砂56井和风砂58井等7口井共22个油砂样品,样品主要涉及下白垩统吐谷鲁组、上侏罗统齐古组,其埋深分别介于95.5~178.1 m、141.8~270.8 m。对所有样品分别进行了饱和烃和芳烃的色谱—质谱分析,首先用正己烷(100 mL)沉淀油砂抽提原油中的沥青质,然后用柱色层把脱沥青质原油分离成饱和烃(正己烷作冲洗剂)、芳香烃(甲苯作冲洗剂)。饱和烃和芳烃的色谱—质谱分析实验条件如下。
饱和烃GC-MS分析:仪器型号为HP-GC 6980/5873MSD。色谱柱为HP-5MS弹性石英毛细柱(30 m×0.125 mm×0.125 μm),以脉冲不分流方式进样,脉冲压力为15 psi,进样温度300 ℃,He为载气,流速为1 mL/min。升温程序如下:初始温度50 ℃,恒温2 min后,以3 ℃/min的速率升温至310 ℃,并维持恒温18 min,EI电离方式,电离能量70 eV,采用内标法对饱和烃进行定量,正构烷烃的标样为C24D50,甾萜类标样为5α-雄甾烷。
芳烃GC-MS分析:仪器型号为HP-GC6890/5973 MSD,色谱柱为HP-5MS(60 m×0.25 mm×0.25 μm),汽化室温度为290 ℃,进样方式是脉冲不分流进样且采用恒流模式,载气流速为1 mL/min。柱炉温的升温程序为:以20 ℃/min升至100 ℃,然后再以3 ℃/min升至310 ℃,恒温18 min。质谱采用EI电离方式,电离能量为70 eV,接口温度为280 ℃,采集方式为全扫描模式,扫描质量范围50~450 amu,采用内标法对芳烃进行定量,标样为D10蒽。
-
目前国内外学者较为统一的原油化合物降解顺序为:正构烷烃<类异戊二烯<甾烷<五环三萜烷<重排甾烷<三环萜烷<三芳甾烷<卟啉[4]。在生物降解初期,正构烷烃会被优先降解,随着主要溶解化合物的减少,色谱基线的驼峰会变得越来越突出,通常将其称为未分离复杂混合物,即UCM鼓包,表示降解强度比较严重(降解强度大于4),而高丰度25-降藿烷是强烈生物降解(生物降解等级大于6)的标志性化合物,其结构与规则藿烷的C26-C34系列化合物相当,是规则藿烷在C-10位失去一个甲基形成的。通常,25-降藿烷出现在那些藿烷被优先消除的原油中。芳香族甾类烃具有很强的抗生物降解能力,只有在极端情况下才会被降解。Wardroper et al.[29]指出C20-C21三芳甾类化合物属于原油生物降解中被最先消耗的芳香甾类化合物,20R构型的单芳甾烷、三芳甾烷会被优先降解,且在严重生物降解8级时单芳甾烷已被完全消耗。
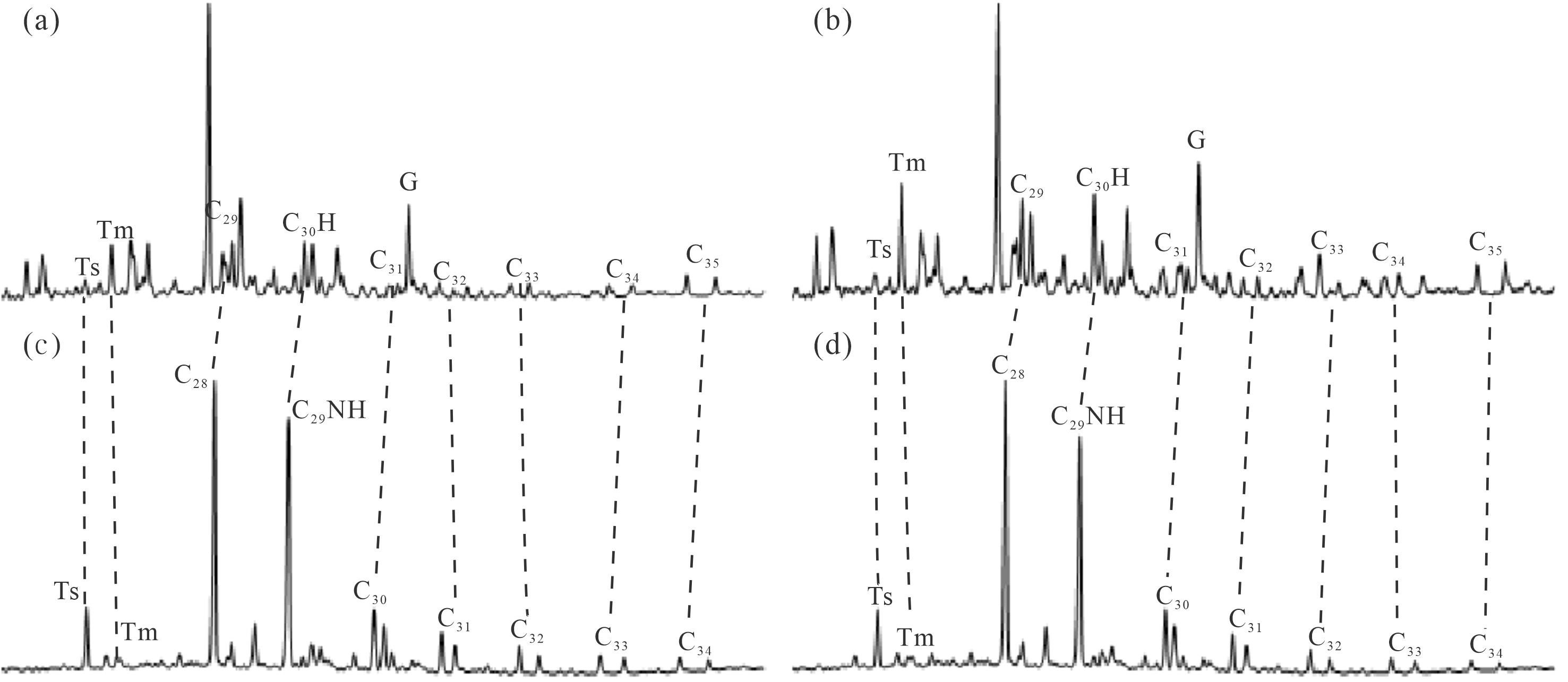
本文样品中均已检测不到正构烷烃、规则甾烷、重排甾烷、单芳甾烷和C20-C21三芳甾烷系列的化合物,保存了高丰度的三环萜烷、藿烷、降藿烷系列化合物,孕甾烷、升孕甾烷,以及长链三芳甾烷系列均有一定量的分布,化合物绝对浓度见表1。其中检测到完整的C28-C34的降藿烷(NH)系列(图1),25-降藿烷(即C29NH)的绝对浓度分布介于687.10~1 240.21 μg/g,相较于C30藿烷(绝对浓度分布范围91.31~912.09 μg/g)和伽马蜡烷(绝对浓度分布范围57.74~667.29 μg/mg),其含量相对较为丰富。根据上述特征初步揭示了研究区原油样品至少经历了8级以上的生物降解作用,属于强烈生物降解的原油。
井号 层位 孕甾烷 伽马蜡烷 C29NH 三芳甾烷 Tm/Ts C20/C19TT TT/H (C20+C21)/C26TT G/C30H C29NH/C30H 风砂53 K1t 348.55 170.45 1 106.51 97.53 4.11 3.68 4.19 0.92 0.99 6.48 风砂52 K1t 0 305.25 917.04 58.83 7.13 4.49 3.02 1.14 1.13 3.39 风砂52 K1t 263.62 61.78 775.90 39.43 4.14 6.13 4.89 0.23 0.68 8.50 风砂54 K1t 372.19 269.47 1 038.53 60.80 6.53 4.39 3.33 0.63 1.57 6.05 风砂51 K1t 290.56 185.47 696.20 43.63 6.32 4.93 3.66 1.59 1.05 3.96 风砂55 K1t 400.68 667.29 687.10 44.11 14.30 5.25 1.40 1.77 0.73 0.75 风砂54 K1t 500.02 365.41 1 146.36 33.82 7.11 6.13 4.39 2.07 1.11 3.47 风砂55 K1t 340.19 185.76 840.52 89.21 6.53 4.25 5.93 0.80 1.17 5.30 风砂51 K1t 417.59 277.94 920.74 76.38 6.68 4.28 3.50 1.43 1.17 3.86 风砂53 K1t 353.38 57.74 1 178.18 78.88 4.01 7.25 4.24 0.26 0.49 10.08 风砂55 J3q 351.21 159.08 901.95 71.62 5.18 5.01 3.67 0.71 1.06 6.00 风砂56 J3q 553.05 299.68 880.01 72.82 11.30 6.22 5.35 3.09 1.37 4.04 风砂53 J3q 506.58 164.59 1 055.60 62.36 0 5.85 8.81 3.66 1.31 8.40 风砂52 J3q 452.72 293.39 790.48 60.74 9.91 5.63 4.66 2.91 1.36 3.66 风砂55 J3q 563.19 187.14 1 240.22 69.26 0 5.89 7.12 3.50 1.16 7.68 风砂56 J3q 584.64 238.83 1 041.31 65.89 0 5.63 6.37 3.58 1.33 5.78 风砂54 J3q 594.80 433.47 994.86 66.83 11.69 5.97 3.17 3.51 0.74 1.69 风砂58 J3q 666.58 479.40 859.59 58.50 14.18 5.89 4.16 1.18 2.49 4.47 风砂51 J3q 594.01 660.44 4 307.56 86.66 0 6.94 2.75 3.40 1.33 8.68 风砂54 J3q 575.33 385.31 749.55 45.05 11.39 6.23 3.79 2.24 1.23 2.39 风砂51 J3q 632.56 326.83 1 077.57 44.03 10.08 6.10 5.37 2.93 1.20 3.95 风砂53 J3q 525.38 151.40 1 176.87 117.93 4.33 6.24 8.48 3.95 1.08 8.38 
Figure 1. Mass chromatogram depicting norhopane of typical oil sand samples in the northwestern margin of Junggar Basin
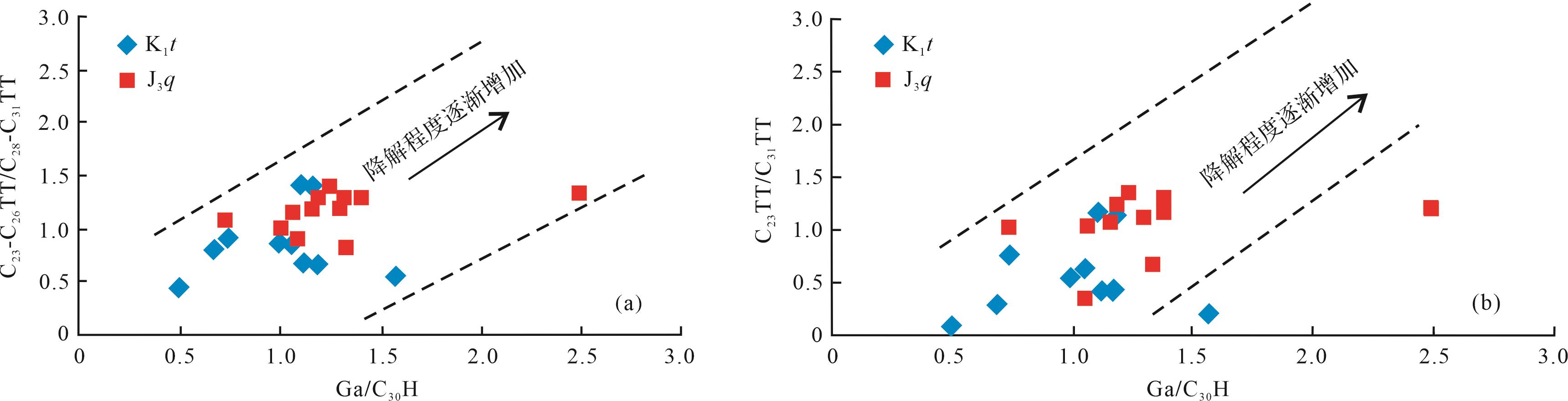
伽马蜡烷、25-降藿烷、孕甾烷及三芳甾烷均为抗生物降解能力较强的化合物,从白垩系样品的伽马蜡烷(绝对浓度均值为254.65 μg/mg,下同)、25-降藿烷(930.71 μg/mg)、孕甾烷(328.68 μg/mg)、三芳甾烷(62.26 μg/mg);侏罗系样品的伽马蜡烷(绝对浓度均值314.96 μg/mg)、25-降藿烷(1256.29 μg/mg)、孕甾烷(550 μg/mg)、三芳甾烷(68.47 μg/mg),可以看出侏罗系样品中抗生物降解能力强的化合物其绝对浓度明显高于白垩系样品。常见的随着生物降解程度增加而增大的生物降解参数(Ts/Tm、C20/C19TT、TT/H、(C20+C21)/C26TT、G/C30H、C29NH/C30H)显示(表1),侏罗系样品的这些参数均大于白垩系样品。
-
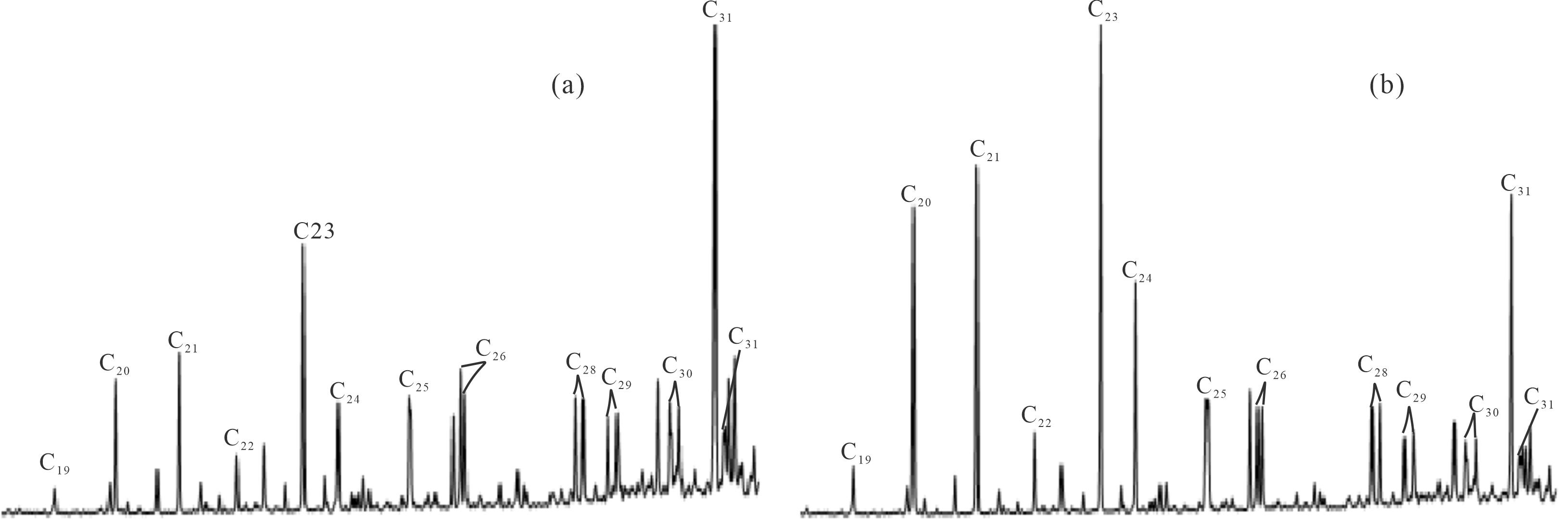
图2为研究区典型原油样品三环萜烷的质量色谱图,总体而言,油砂样品中分布有较为完整的C19-C31三环萜烷系列化合物(缺失C27)。白垩系样品与侏罗系样品中三环萜烷的分布特征明显不同,白垩系样品抽提物主要以C31三环萜烷为主峰,而侏罗系样品抽提物中则以C23三环萜烷为主峰。此外,侏罗系油砂抽提物中C20、C21三环萜烷的丰度均比白垩系高。本文在计算油砂样品三环萜烷系列化合物的绝对浓度时,发现侏罗系样品中三环萜烷总浓度普遍高于白垩系样品。准噶尔盆地西北缘三环萜烷含量异常丰富,碳数范围较大,高碳数三环萜烷含量也较高,这与文献报道的研究结果相一致[30]。
-
ETR=(C28+C29)/(C28+C29+Ts)最早是由Holba et al.[31]提出用来划分三叠系与侏罗系的参数,之后有学者发现ETR可作为指示海侵作用或盐度和碱度的指标[32-33],也有学者在研究强烈生物降解原油时发现ETR会随着生物降解程度的增加而增大[34-35]。研究区样品中ETR与三环萜烷的浓度呈较好的正相关关系(图3),且侏罗系样品中的ETR普遍高于白垩系样品。该关系证实了侏罗系油砂样品的原油生物降解程度高于白垩系样品,反之也表明三环萜烷越丰富,生物降解作用越强。
-
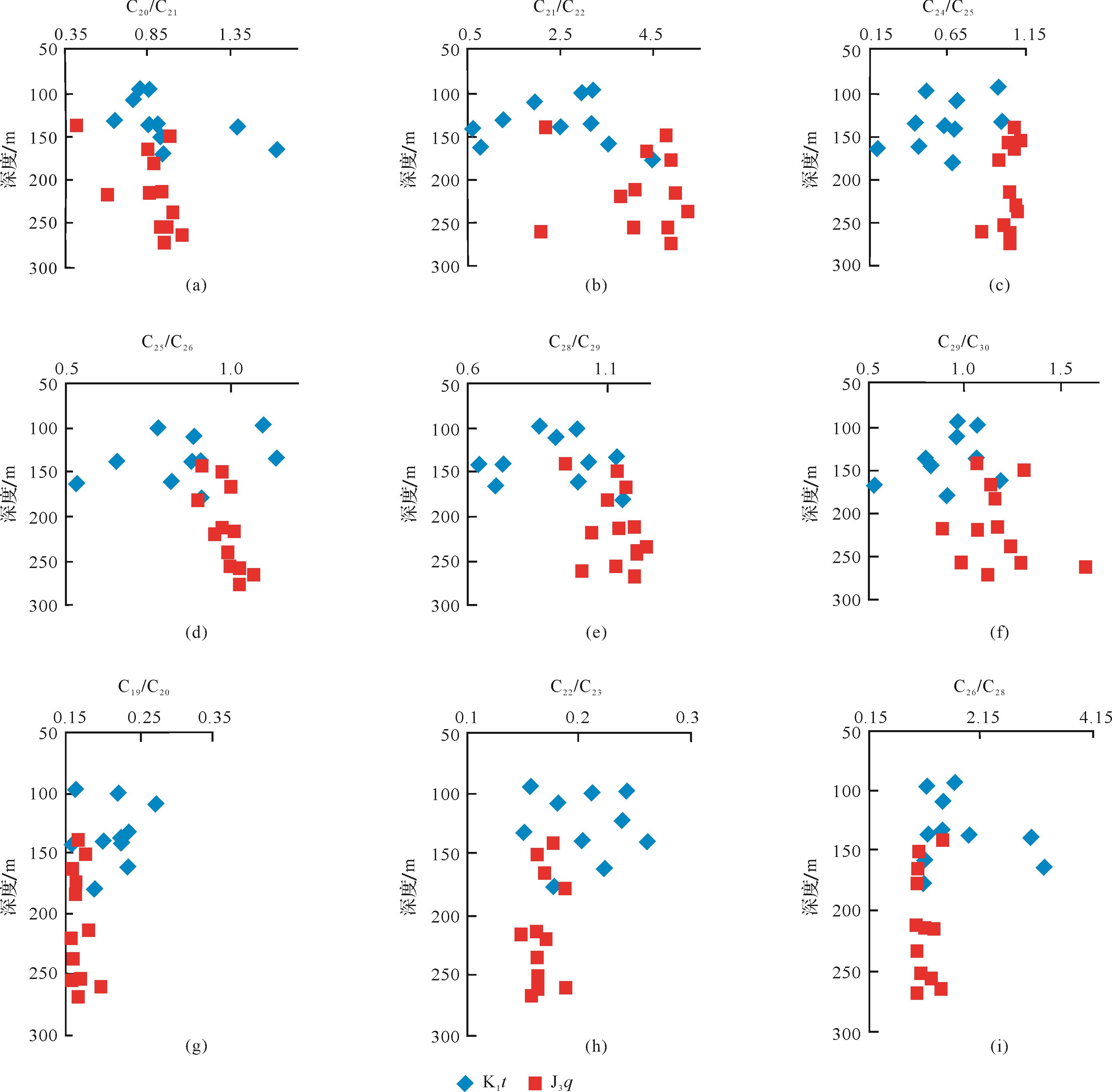
一般而言,原油遭受生降解的程度是随着深度的增加而逐渐降低的。本文在分析一些抗生物降解能力强的化合物浓度及其相关比值时发现,侏罗系油砂样品的生物降解程度明显高于白垩系样品,即使侏罗系油砂样品的深度大于白垩系样品。在此基础上,试图通过相邻碳数三环萜烷的比值与深度的分布关系来刻画不同碳数的三环萜烷的抗降解能力(图4)。可以看出C20/C21TT、C21/C22TT、C24/C25TT、C25/C26TT、C28/C29TT、C29/C30TT比值与深度呈较好的相关关系,表明这些比值是随着降解程度的增加而增加的,由此可知在C20-C22TT、C24-C26TT、C28-C30TT这三个范围内的三环萜烷是随着碳数的增加,其抗降解能力逐渐减弱;而C19/C20TT、C22/C23TT、C26/C28TT这些比值与深度呈反相关关系,即C20TT抗降解能力要高于C19TT,C23TT抗降解能力高于C22TT,C26TT抗降解能力高于C28TT。根据上述规律,以C22TT、C26TT为界,将研究区样品中C19-C31(除C27)三环萜烷的分布划分为低碳数、中碳数、高碳数三个部分:其中C19-C22TT为低碳数,C23-C26TT为中碳数,C28-C31TT为高碳数。并根据上述分类来探讨下文高、中、低碳数的三环萜烷之间的生物降解程度。

Figure 4. Relationship between different carbon number tricyclic terpanes and depth of oil sands in the northwestern margin of Junggar Basin
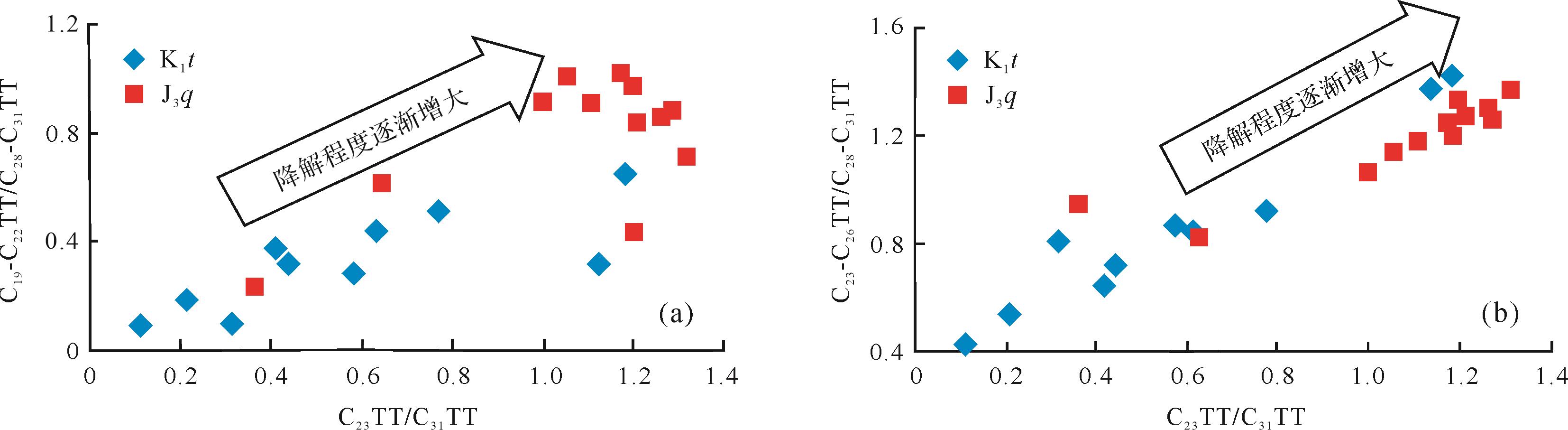
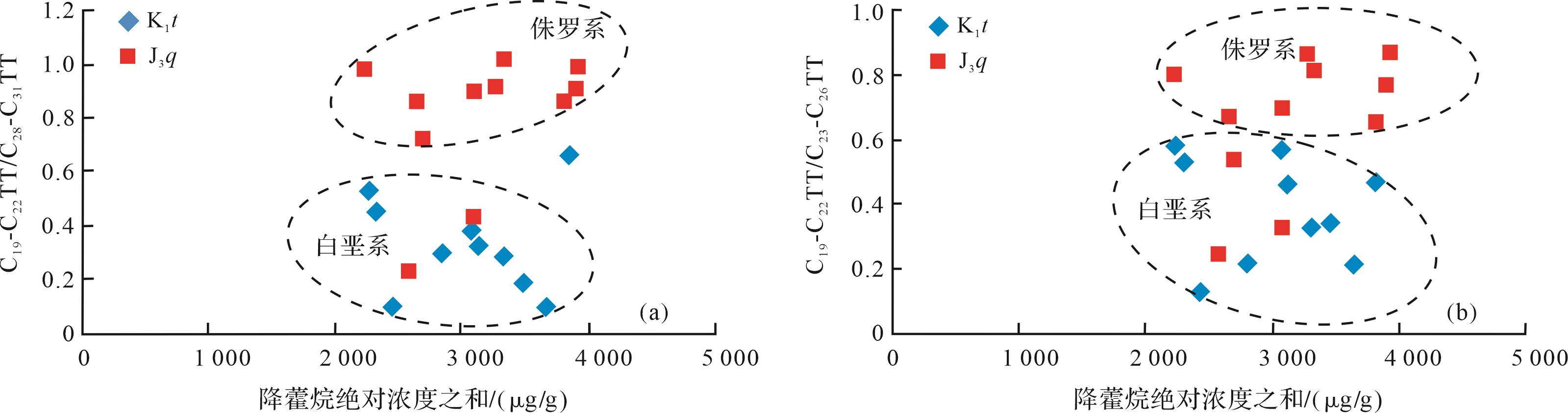
侏罗系的油砂样品的生物降解程度高于白垩系样品,并且侏罗系油砂样品的抽提物中以C23TT为主峰,白垩系油砂样品抽提物以C31TT为主峰,故C23/C31TT可以表示随生物降解程度的增加,该比值也是增加的。从图5中可以看出,C19-C22TT/C28-C31TT、C23-C26TT/C28-C31TT比值均与C23/C31TT比值呈较好的正相关关系。良好的正相关关系也说明随着降解程度的增加,C19-C22TT/C28-C31TT、C23-C26TT/C28-C31TT的比值在增大,由此推断低碳数和中碳数的三环萜烷的抗生物降解能力是强于高碳数三环萜烷的。

Figure 5. Correlation between the ratios of C23TT/C31TT and C19⁃C22TT/C28⁃C31TT, C23⁃C26TT/C28⁃C31TT of oil sandsin the northwestern margin of Junggar Basin
低碳数、中碳数和高碳数三环萜烷的相对百分含量关系如图6所示,白垩系的样品点更靠近C28-C31TT,而侏罗系样品点更靠近C19-C22TT。由前文分析可知,侏罗系样品遭受生物降解的程度高于白垩系样品,据此推断研究区三环萜烷化合物随着碳数的增加,其抗生物降解的能力是逐渐减弱的,即三环萜烷抗生物降解能力为高碳数(C28-C31TT)<中碳数(C23-C26TT)<低碳数(C19-C22TT)。
-
伽马蜡烷比藿烷抗生物降解能力要强[36]。伽马蜡烷指数(Ga/C30H)会随着生物降解程度的增强而增大。伽马蜡烷指数与C23-C26TT/C28-C31TT、C23TT/C31TT比值的关系显示(图7),随着伽马蜡烷指数的增加,C23-C26TT/C28-C31TT、C23TT/C31TT比值也随之增大,表明C23-C26TT/C28-C31TT、C23TT/C31TT比值随着生物降解程度的增强而增大。二者之间良好的线性关系也说明在受到强烈生物降解作用影响时,伽马蜡烷指数仍然可以用来判断受到强烈生物降解作用的样品的降解程度。然而,伽马蜡烷易受其他因素的影响,据前人研究[36],准噶尔盆地西北缘油砂的地化特征总体上较为相似,三环萜烷极其丰富而藿烷含量低,其油源来自南部的玛湖凹陷,属于同一类原油,故此处用伽马蜡烷来判断生物降解的等级是有意义的。
-
藿烷被生物降解时常产生一些新的化合物,包括C26和C29-C3417α(H),21β(H),25-降藿烷,这一系列通常被认为是发生严重生物降解的原油中的典型生物标志物[37-38]。一般而言,17α(H),21β(H)-25-降藿烷/C30H的比值随着生物降解作用的增强而增大。研究区原油样品普遍遭受了强烈的生物降解作用,对比研究研究区降藿烷系列化合物相关参数与三环萜烷相关比值,并未发现降藿烷系列化合物相关参数有明显的变化趋势。这表明在研究受到强烈生物降解作用地区的样品时,这些指标参数可能已经失效。因此,本文对研究区降藿烷化合物绝对浓度也进行了研究。降藿烷绝对浓度之和与C19-C22TT/C23-C26TT、C19-C22TT/C28-C31TT比值的关系显示(图8),C19-C22TT/C23-C26TT、C19-C22TT/C28-C31TT比值可有效划分研究区两种受到不同生物降解作用的样品;而降藿烷的绝对浓度虽然很高,但是在侏罗系、白垩系的样品分布均在较为相似的分布范围,不能有效划分遭受过强烈生物降解作用影响的样品。因此认为降藿烷系列化合物的绝对浓度与相对比值已不能用来判断受到强烈生物作用的程度,在选用降藿烷系列化合物分析受到强烈生物降解地区的样品时,需慎重选择相关降藿烷类参数。
-
Anders et al.[11]认为C20-C25三环萜烷是五环三萜的降解产物,而Neto et al.[39]则认为三环萜烷与藿烷并没有联系。根据藿烷的生物降解途径,程熊等[24]提出3种三环萜烷的降解途径:1)三环萜烷支链末端的甲基被氧化而形成三环萜烷酸;2)三环萜烷直接脱去C-10位的甲基而形成脱甲基三环萜烷;3)先发生途径一生成三环萜烷酸,再由途径二形成的脱甲基三环萜烷酸发生脱羧反应从而形成脱甲基三环萜烷。本文样品中并未检测到脱甲基三环萜烷,说明三环萜烷是在未进行脱甲基的情况下形成的,故认为研究区三环萜烷的形成途径也是三环萜烷支链末端的甲基被氧化而形成的。Neto et al.[39]分析发现C20+三环萜烷是在C-14位处包含一个正的类异戊二烯侧链,C22、C27三环萜烷的丰度一般都比较低或者没有,表明这个位置有分支或者侧链异构体。Peters[40]研究发现C32三环萜烷在所有样品中的含量都很低,因为它们需要两个碳碳键的裂解才能从更高的同源物中形成,由此猜测低碳数的三环萜烷需要从更高的同源物中碳碳键的裂解才能形成。C20+三环萜烷的形成是在C-14位连接了一个侧链,侧链被微生物侵蚀完全时只剩下C20三环萜烷;而C19三环萜烷则是C-13位处连接了一个甲基,所以C20三环萜烷会比C19三环萜烷更容易形成,也反映了C20三环萜烷比C19三环萜烷更抗生物降解。
Anders et al.[11]研究发现,C19-C26三环萜烷(除C20外)的抗生物降解能力是随着碳数的增加而逐渐增强的。但有原油生物降解模拟实验定量分析了C19-C29三环萜烷,结果表明高碳数(C25+)三环萜烷抗生物降解能力高于低碳数(C25-)[41]。结合本次研究,在遭受强烈生物降解作用时,只有C19与C20、C22与C23、C26与C28三环萜烷的抗生物降解能力有明显随碳数增加而增加的趋势,而在C20-C22TT、C24-C26TT、C28-C30TT三环萜烷这几个区域内的三环萜烷随着碳数的增加,抗降解能力是逐渐减弱的。因此,推测三环萜烷化合物的抗生物降解能力与样品受到的降解程度有关,而不是一个固定的降解序列。
3.1. 生物降解强度的确定
3.2. 三环萜烷内组成特征
3.2.1. 三环萜烷碳数分布特征
3.2.2. ETR参数分布特征
3.2.3. 三环萜烷不同碳数化合物比值分布特征
3.3. 三环萜烷化合物与其他化合物的对比
3.3.1. 伽马蜡烷
3.3.2. 降藿烷
3.4. 讨论
-
(1) 准噶尔盆地西北缘油砂样品中,正构烷烃、类异戊二烯烷烃、常规甾烷已经蚀变检测不到,藿烷类化合物、孕甾烷、升孕甾烷及长链三芳甾烷少量分布;三环萜烷绝对浓度异常丰富,有较完整的25-降藿烷系列化合物分布且绝对浓度含量较高,判断研究区原油曾遭受过强烈的生物降解作用,降解等级为8级以上。
(2) ETR参数揭示了研究区侏罗系样品的降解程度稍高于白垩系。通过各碳数比值与深度的关系研究,将三环萜烷化合物碳数划分为低碳数(C19-C22)、中碳数(C23-C26)和高碳数(C28-C31)。研究表明随着生物降解作用的增强,三环萜烷化合物的抗降解能力会随着碳数的增加而减弱,即三环萜烷抗生物降解能力为高碳数(C28-C31TT)<中碳数(C23-C26TT)<低碳数(C19-C22TT)。
(3) 降藿烷系列化合物和伽马蜡烷指数与C19-C22TT/C28-C31TT、C23-C26TT/C28-C31TT、C23TT/C31TT做了相关的对比研究,发现随着生物降解程度的增加,C19-C22TT/C28-C31TT、C23-C26TT/C28-C31TT、C23TT/C31TT、C19-C22TT/C23-C26TT的比值均随之增大;且伽马蜡烷指数与这些指标均具有较好的相关性,而降藿烷系列化合物的绝对浓度与相对比值已不能用来判断受到强烈生物降解的样品,因此在选用降藿烷化合物相关参数来判断受到强烈生物降解作用地区的样品时,需要慎重选择。











 DownLoad:
DownLoad: